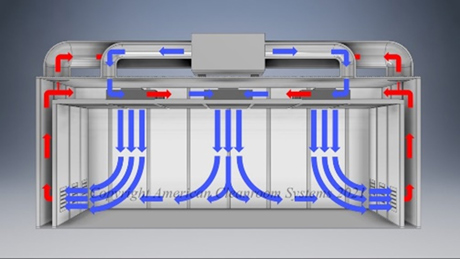
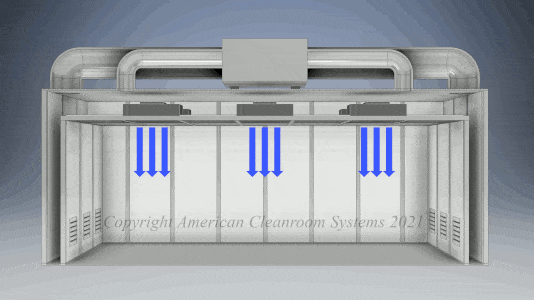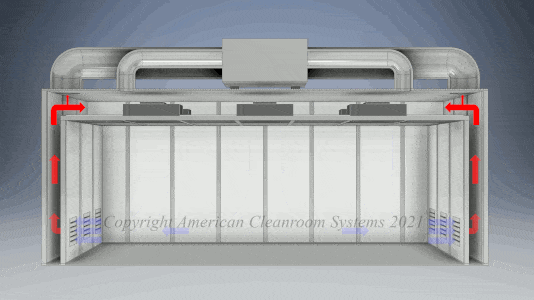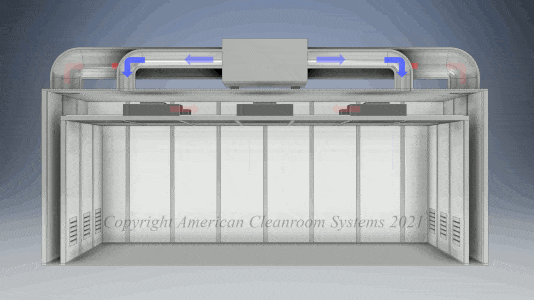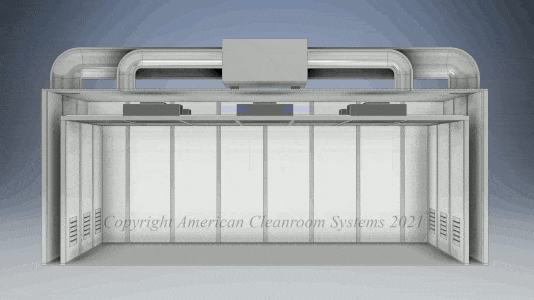
A: Pharmaceutical manufacturers are subject to FDA validation of their manufacturing which typically specify use of a clean room to ensure the quality of the manufactured pharmaceutical product. Sterility is highest priority. Pharmaceutical cleanrooms focus on both non viable (inanimate) and viable (live) contamination. They typically use laser particle counters to measure non viable contamination levels and settling plates with culture media to measure viable contamination levels. Pharmaceutical cleanrooms use aggressive chemical and UV light cleaning techniques to maintain sterility.
A: Cleanrooms are used in any industry that wants to control contamination in their facility. It is common to see pharmaceutical cleanrooms, medical device cleanrooms, semiconductor cleanrooms, electronic cleanrooms, aerospace cleanrooms, food cleanrooms, USP797 compounding pharmacy cleanrooms and biotech cleanrooms. Cleanrooms are also used by the government such as national labs, defense industries, and R&D labs at universities.
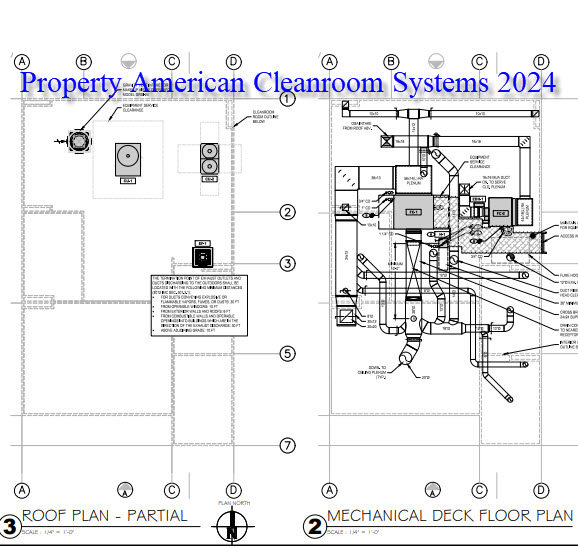
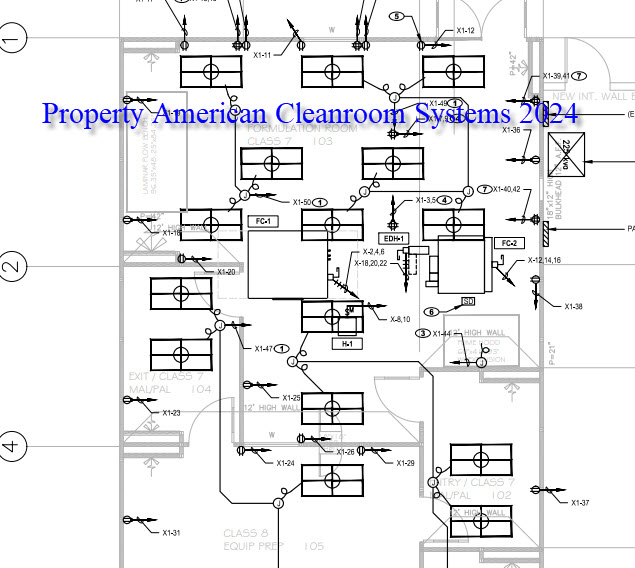
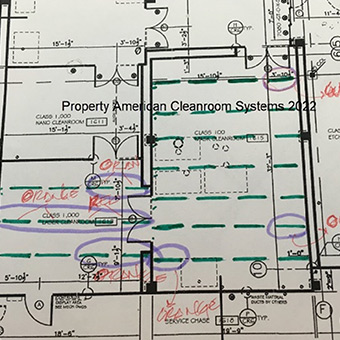
A: Most common cleanroom requirements include cleanroom classification, size, number of rooms, cleanroom flooring, cleanroom ceiling height, cleanroom chemical resistance, and cleanroom temperature and humidity.