949.589.5656
ESCIBENOS A: INFO@AMERICANCLEANROOMS.COM PARA EMPEZAR
- Inicio
- Quiénes Somos
- Planificación
- Clasificaciones de Cuartos Limpios
- Diseño de cuarto limpio
- Ingeniería de Cuartos Limpios
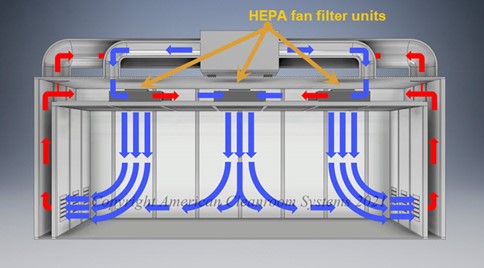
- Flujo de aire en el cuarto limpio
- Sistemas de Pared Para Cuarto Limpio
- Pisos de Cuartos Limpios
- Accesorios para Cuarto Limpio
- Equipo Para Cuarto Limpio
- Techos de Cuarto Limpio
- Sistema de Monitoreo CGMP para cuarto limpio
- Visite Nuestro Cuarto Limpio De Muestra
- Fabricación
- Certificación
- Cuarto Limpio
- Cuartos Limpios Completamente Preconfigurados
- Cuarto limpio CGMP
- Cuartos Limpios Modulares
- Cuartos Limpios de Dispositivos Médicos
- Cuarto limpio para fabricación de mascarillas
- Cuartos Limpios Softwall
- Cuartos Limpios para CBD/ Extracción
- E-Líquido
- Cuartos Limpios Antiestáticos
- Cuartos Limpios para Láseres
- Oficinas y laboratorios modulares
- Cuartos limpios farmacéuticos
- Cuarto limpio híbrido
- Cuarto Limpio LEED
- USP797 USP800 Cuarto Limpio
- Cuartos limpios de bahía alta
- Proyectos
- Testimonios
- Noticias
- Cleanroom Blogs
- Videos